Researchers soon figured out the cause of the ozone hole. Long-lasting extreme cold in the Antarctic stratosphere in winter created the perfect conditions for chemical reactions that destroy ozone at a much faster pace than those that take place elsewhere in the atmosphere. When temperatures are very low in the stratosphere, nitric acid and water can turn from gas into liquid or solid forms, resulting in a wispy multicolored cloud called a polar stratospheric cloud. These frozen particles of acid and water provide surfaces on which the chemical reactions that convert chlorine into ozone-destroying forms can take place.
Michelle Santee, another JPL researcher studying the Arctic ozone loss, said, “If you just had gaseous molecules, as is the case in most of the atmosphere, these reactions would not occur.”
Measuring Arctic ozone loss
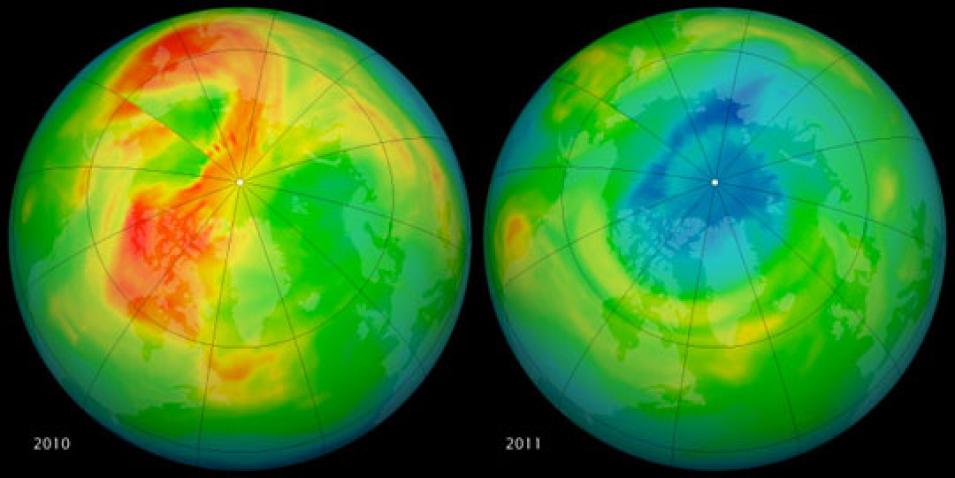
Outside of Antarctica, stratospheric temperatures rarely get low enough for polar stratospheric clouds to form, nor do they typically stay low long enough for chlorine to persist in ozone-destroying forms. So when Santee and Manney saw a large decrease in ozone over the Arctic during routine inspection of data from NASA's Microwave Limb Sounder (MLS), they wondered how bad the problem was. MLS data archived at NASA's Goddard Earth Sciences Data and Information Services Center (GES DISC) showed that about 80 percent of the ozone at 18 to 20 kilometers altitude had been destroyed—far more ozone loss than ever previously seen over the Arctic.
Confirming the MLS data, balloon-borne ozonesondes released every year over the Arctic provide a high-resolution series of data dating back to the 1990s, before the MLS record began; balloons have also provided measurements of ozone over the Antarctic since the 1980s. Manney said, “These comparisons were important in establishing that the ozone loss in 2011 was unprecedented in the Arctic, and that it was comparable to that in some Antarctic ozone holes.”
But while MLS and the balloon-borne ozonesondes measure ozone at different altitudes, they do not provide a measurement of the total ozone column. Manney said, “If you’re standing on the surface of the Earth, worried about getting a sunburn, what you really want to know is the total amount of ozone overhead.” For that information they turned to Levelt and her colleagues who work with OMI, which measures the vertical column of ozone. Levelt said, “The definition of whether an ozone hole is present or not has historically been based on total column measurements.”
The OMI data, also archived at GES DISC, showed extremely low total ozone values over a much larger region than had ever been seen in the Arctic since satellite measurements started in 1979. However, the ozone loss was not as severe as that in the annual Antarctic ozone hole, which has grown in size and intensity over the last twenty years. Manney said, “The amount of ozone that was destroyed was comparable to what we saw a couple of decades ago in the Southern Hemisphere, when we first started studying the Antarctic ozone hole.”
Manney and Santee also needed to know whether the ozone loss was caused by chlorine-containing chemicals, and whether polar stratospheric clouds were present in the Arctic atmosphere. Temperatures in the Arctic stratosphere had been unusually low, which meant that the polar stratospheric clouds that catalyze ozone destruction were likely to form. Data from the Cloud Aerosol Lidar with Orthogonal Polarization (CALIOP) sensor on the NASA/French Space Agency Cloud-Aerosol Lidar and Infrared Pathfinder Satellite Observation (CALIPSO) satellite, archived at NASA's Atmospheric Science Data Center (ASDC), confirmed the team’s suspicions, showing that polar stratospheric clouds were indeed present during the time ozone values were decreasing.
Together, the data gave a comprehensive picture of the conditions that led to the unprecedented Arctic ozone depletion. “The data from all of these instruments played vital roles; they all provided pieces of the puzzle,” Santee said.