The measure of life and death
Typical measurements of fossil fuel emissions look at fuel consumption, and the potential carbon dioxide (CO2) within that fuel. On a global scale, accuracy can be within 5 percent, but testing fossil fuel emission on a regional scale requires isolating factors that easily cross arbitrary borders, such as wind blowing across California state lines.
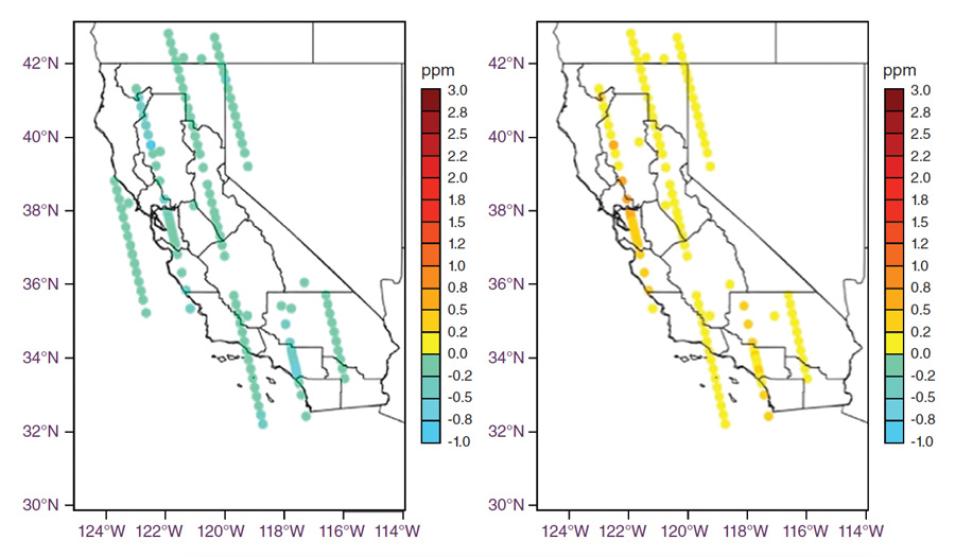
By modeling the motion of air over California in fine detail, the researchers could observe where air came from, how it moved, and how it interacted with the environment. “We’re basically looking at the difference between what’s in California and what’s flowing into California as a way of judging what California must have added or subtracted,” said Marc Fischer, an atmospheric scientist at Lawrence Berkeley National Laboratory.
Only four hundredths of a percent of air is composed of CO2, a heat-trapping greenhouse gas. Methane (CH4) warrants equal attention as an emission source from cows, landfills, and gas leaks, but limitations in satellite instrumentation, which have yet to detect methane, led the researchers to focus solely on CO2 emissions.
All life respires, releasing CO2 into the atmosphere, but plants do more. “Plants are supremely beautiful living organisms because they are both breathing in CO2 during the day and breathing it out at night,” Fischer said. During photosynthesis, plants absorb CO2 and sunlight to create fuel—glucose and other sugars—to build plant structures. This accumulation of CO2 as biomass in plants removes CO2 from the atmosphere.
Measuring CO2 in the biosphere, where exchanges occur between living organisms, gets complicated because life is dynamic. Seasons shift, droughts happen, and plant metabolism decreases. To differentiate between fossil fuel emissions, which also spew CO2 into the atmosphere, the team traced the origins of the carbon molecules. All life, living and long dead as with fossil fuels, is composed of carbon. Carbon holds six protons, but can have a different number of neutrons in its nuclei, thus changing its mass to become an isotope. Carbon has two stable isotopes, 12C and 13C, and the unstable, radioactive 14C, also called radiocarbon, with a half-life of 5,700 years.
When solar particles smack into the upper atmosphere, they form a tiny, continuous amount of 14C, which plants incorporate through photosynthesis and animals by eating plants. When an animal dies, carbon is no longer exchanged with its environment, and radiocarbon begins to decay. The older a sample, the less radiocarbon it carries. Fossil fuels, or decayed organic matter that has been underground for millions of years, contain no radiocarbon. So a big drop of radiocarbon in the atmosphere indicates a rise in fossil fuel emissions.