Dr. Emily Fischer; Associate Professor, Department of Atmospheric Science and School of Global Environmental Sustainability; Colorado State University, Fort Collins, CO
Research interests: Using satellite observations, ground-based and airborne measurements, and chemical transport models to describe the sources, fate, and impacts of pollutants in lower levels of Earth’s atmosphere.
Research highlights: Ozone (written chemically as “O3” to designate that it is made up of three oxygen atoms) is a pretty amazing molecule. It is highly reactive, which means it easily can be broken into components of individual molecules of atomic oxygen (O) or into molecular oxygen (O2) and then re-combine back into ozone (O3). This molecular breaking and re-combining is caused primarily by ultraviolet (UV) radiation from the Sun, and is rapidly and constantly occurring in a layer of the atmosphere called the stratosphere, which extends from about 10 to 50 km above Earth’s surface. Through this process, most of the Sun’s harmful UV radiation is absorbed and prevented from reaching lower levels of the atmosphere (check out the frightening results of a NASA model simulation predicting what could happen if Earth’s stratospheric ozone were depleted). Fortunately for life on Earth, 90 percent of atmospheric ozone is in the stratosphere.
The remaining 10 percent of Earth’s ozone resides in the lowest layer of the atmosphere, called the troposphere, which extends from the surface to about 10 km. This is where the whole breaking and recombining of ozone molecules becomes more complex—and more interesting for researchers like Dr. Emily Fischer. Using satellite-derived Earth observing data from NASA’s Earth Observing System Data and Information System (EOSDIS) collection combined with model data and data acquired through field investigations and aircraft-borne instruments, Fischer and her colleagues are exploring how a trace gas called peroxyacetyl nitrate (commonly known as “PAN”) impacts the distribution of ozone in the troposphere.
In the lower atmosphere, PAN and ozone are two important pollutants, particularly in and around urban areas. Both are poisonous to plants in high concentrations, and both can affect human health. PAN is a strong eye and respiratory irritant, and ground-level ozone can cause a wide range of respiratory issues. As noted by the U.S. Environmental Protection Agency (EPA), “long-term exposure to [low-level] ozone is linked to aggravation of asthma, and is likely to be one of many causes of asthma development. Long-term exposures to higher concentrations of ozone may also be linked to permanent lung damage.”
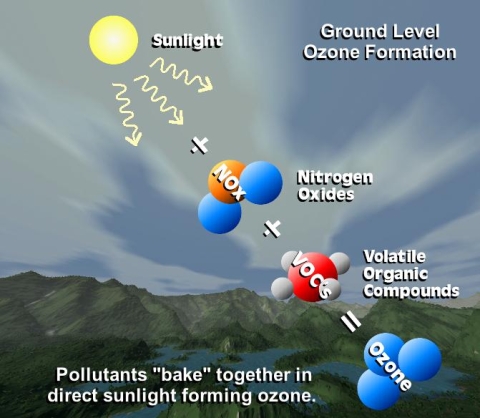
Tropospheric ozone forms in a different way than stratospheric ozone. In the troposphere, nitrogen dioxide (NO2) rather than molecular oxygen (O2) is the primary source of the oxygen atoms required for ozone formation. Sunlight splits nitrogen dioxide (NO2) into nitric oxide (NO) and an oxygen atom (O), and this oxygen atom combines with molecular oxygen to form ozone (O + O2 = O3).
Nitrogen dioxide (NO2) and nitric oxide (NO) are together known as NOx (pronounced knocks). NOx plays a critical role in many chemical cycles occurring in lower levels of the atmosphere.
NOx is formed when nitrogen and oxygen react with each other during combustion at high temperatures. Fossil fuel combustion is the principal source of NOx, but there are additional contributions from biomass burning, lightning, and soils. NOx can cause serious health problems to humans, including respiratory diseases, and is responsible for smog and the “brown cloud” that can cover large cities and produce poor air quality. NOx emissions contribute to acid rain and the formation of ground-level ozone.
Which brings us back to PAN. As noted by Fischer and her colleagues, PAN is the most important reservoir for NOx in the troposphere and plays a significant role in the redistribution of NOx to remote regions. This, in turn, has major implications for the global distributions of tropospheric ozone and other pollutants.
Much of the satellite data used by Fischer in her PAN research were acquired by the Tropospheric Emission Spectrometer (TES) instrument aboard NASA’s multi-national Aura satellite. Launched in 2004, Aura’s mission is to acquire measurements of ozone, aerosols, and key gases throughout the atmosphere.
TES collected data between 2004 and its decommissioning in 2018 due to a part failure. Routine standard data products from TES include vertically-resolved profiles for atmospheric ozone (O3), carbon monoxide (CO), water vapor (H2O), and methane (CH4). TES data are also turned into specially processed data products, including atmospheric profiles of ammonia (NH3) and PAN.
TES data are available through NASA’s Atmospheric Science Data Center (ASDC), which is the EOSDIS Distributed Active Archive Center (DAAC) responsible for EOSDIS data related to Earth’s radiation budget, clouds, aerosols, and tropospheric composition. As noted by Fischer in a NASA news release about the TES decommissioning, “TES really paved the way in our global understanding of both PAN and [ammonia], two keystone species in the atmospheric nitrogen cycle.”
Fischer observes that PAN is difficult to measure, and there are large gaps in our knowledge of the distribution, seasonal cycles, and interannual variability of PAN. She was part of a team that worked with researchers at NASA’s Jet Propulsion Laboratory (JPL) in Pasadena, California, to analyze TES-derived tropospheric measurements of PAN that showed how boreal fires are a key source of interannual variability in reactive nitrogen during the spring in high latitudes. She and her colleagues also confirmed that TES is sufficiently sensitive to detect PAN enhancements several days downwind of fires over North America (biomass burning, such as through wildfires, is a significant source of nitrogen).
The next step in Fischer’s research into biomass burning and PAN will be to analyze PAN data from the Cross-Track Infrared Sounder (CrIS) instrument aboard the joint NASA/NOAA Suomi National Polar-orbiting Partnership satellite (Suomi NPP; operational 2011 to present). Along with providing detailed atmospheric temperature and moisture observations, CrIS also measures atmospheric chemistry and can detect the concentration of atmospheric greenhouse gases.

Fischer’s objective is to integrate CrIS data with data from a recent National Science Foundation (NSF)-funded airborne campaign on which she was the lead Principal Investigator (PI). The campaign, called the Western Wildfire Experiments for Cloud Chemistry, Aerosol Absorption, and Nitrogen (WE-CAN), took place in the summer of 2018 and used an instrument-laden National Center for Atmospheric Research (NCAR) C-130 aircraft to better understand the impact of Western wildfires on PAN and ozone over North America during summer months. Fischer’s objective is to harness PAN satellite data from both TES and CrIS to close knowledge gaps in our understanding of the sources of NOx in remote regions of the troposphere.
The chemical ingredients of the lower atmosphere come together using recipes that are slowly being deciphered by researchers like Fischer. The shopping list for these ingredients come from Earth observing data, many of which are part of NASA’s EOSDIS collection.
Representative data products and models used:
- TES/Aura L2 Peroxyacyl Nitrate Lite Nadir V007 (TL2PANLN; doi:10.5067/AURA/TES/TL2PANLN.007); available through NASA's ASDC
- Goddard Earth Observing System Chemistry-Climate Model (GEOS-CCM); available through the Global Modeling and Assimilation Office at NASA’s Goddard Space Flight Center in Greenbelt, Maryland
- CrIS data, available through NASA’s Goddard Earth Sciences Data and Information Services Center (GES DISC)
- WE-CAN campaign data; available through NCAR
Read about the research:
Fischer, E.V., Zhu, L., Payne, V.H., Worden, J.R., Jiang, Z., Kulawik, S.S., Brey, S., Hecobian, A., Gombos, D., Cady-Pereira, K. & Flocke, F. (2018). Using TES retrievals to investigate PAN in North American biomass burning plumes. Atmospheric Chemistry and Physics, 18(8): 5639-5653. doi:10.5194/acp-18-5639-2018
Rasmussen, C. (2018). Farewell to a Pioneering Pollution Sensor. NASA’s Earth Science News Team. Published online February 13, 2018. Available online (link).
Payne, V.H., Fischer, E.V., Worden, J.R., Jiang, Z., Zhu, L., Kurosu, T.P. & Kulawik, S.S. (2017). Spatial variability in tropospheric peroxyacetyl nitrate in the tropics from infrared satellite observations in 2005 and 2006. Atmospheric Chemistry and Physics, 17(10): 6341- 6351. doi:10.5194/acp-17-6341-2017
Zhu, L., Payne, V.H., Walker, T.W., Worden, J.R., Jiang, Z., Kulawik, S.S. & Fischer, E.V. (2017). PAN in the Eastern Pacific Free Troposphere: A Satellite View of the Sources, Seasonality, Interannual Variability and Timeline for Trend Detection. Journal of Geophysical Research Atmospheres, 122(6): 3614-3629. doi:10.1002/2016JD025868
Fischer, E.V., Jacob, D.J., Yantosca, R.M., Sulprizio, M.P., Millet, D.B., Mao, J., Paulot, F., Singh, H.B., Roiger, A., Ries, L., Talbot, R.W., Dzepina, K. & Pandey Deolal, S. (2014). Atmospheric peroxyacetyl nitrate (PAN): a global budget and source attribution. Atmospheric Chemistry and Physics, 14(5): 2679-2698. doi:10.5194/acp-14-2679-2014
Payne, V.H., Alvarado, M.J., Cady-Pereira, K.E., Worden, J.R., Kulawik, S.S. & Fischer, E.V. (2014). Satellite observations of peroxyacetyl nitrate from the Aura Tropospheric Emission Spectrometer. Atmospheric Measurement Techniques, 7(11): 3737-3749. doi:10.5194/amt-7-3737-2014
Zhu, L., Fischer, E.V, Payne, V.H., Worden, J.R. & Jiang, Z. (2015). TES Observations of the Interannual Variability of PAN over Northern Eurasia and the Relationship to Springtime Fires. Geophysical Research Letters, 42(17): 7230-7237. doi:10.1002/2015GL065328