Dr. Emily Fischer; Associate Professor, Department of Atmospheric Science and School of Global Environmental Sustainability; Colorado State University, Fort Collins, CO
Research interests: Using satellite observations, ground-based and airborne measurements, and chemical transport models to describe the sources, fate, and impacts of pollutants in lower levels of Earth’s atmosphere.
Research highlights: Ozone (written chemically as “O3” to designate that it is made up of three oxygen atoms) is a pretty amazing molecule. It is highly reactive, which means it easily can be broken into components of individual molecules of atomic oxygen (O) or into molecular oxygen (O2) and then re-combine back into ozone (O3). This molecular breaking and re-combining is caused primarily by ultraviolet (UV) radiation from the Sun, and is rapidly and constantly occurring in a layer of the atmosphere called the stratosphere, which extends from about 10 to 50 km above Earth’s surface. Through this process, most of the Sun’s harmful UV radiation is absorbed and prevented from reaching lower levels of the atmosphere (check out the frightening results of a NASA model simulation predicting what could happen if Earth’s stratospheric ozone were depleted). Fortunately for life on Earth, 90 percent of atmospheric ozone is in the stratosphere.
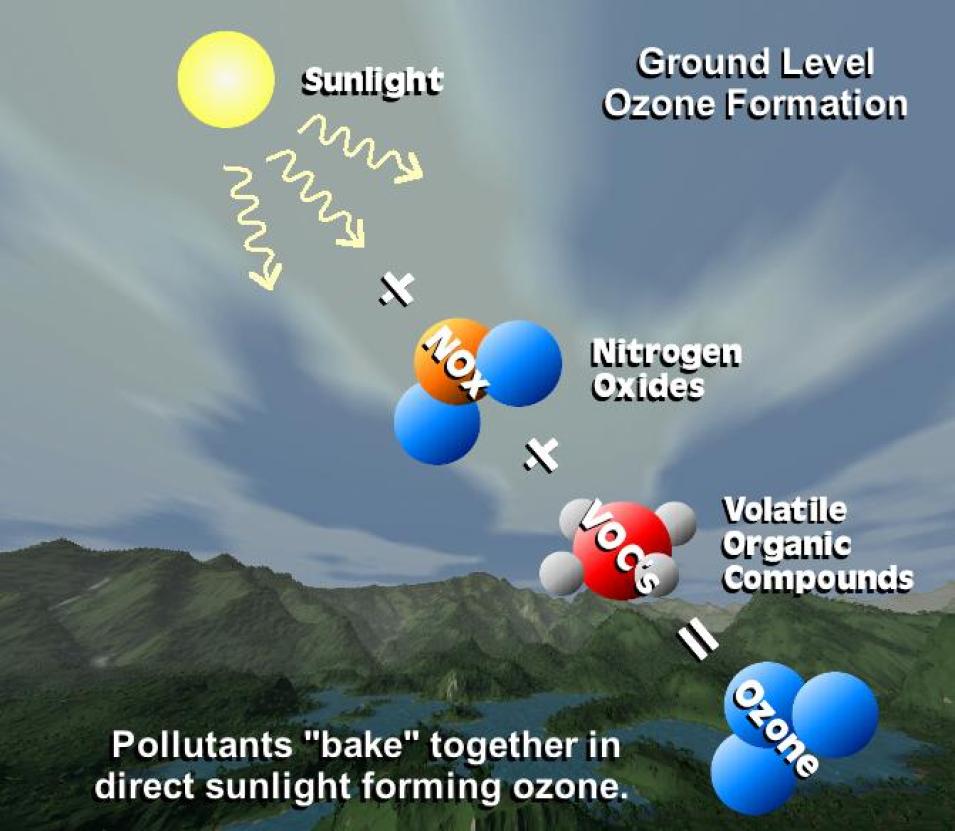
The remaining 10 percent of Earth’s ozone resides in the lowest layer of the atmosphere, called the troposphere, which extends from the surface to about 10 km. This is where the whole breaking and recombining of ozone molecules becomes more complex—and more interesting for researchers like Dr. Emily Fischer. Using satellite-derived Earth observing data from NASA’s Earth Observing System Data and Information System (EOSDIS) collection combined with model data and data acquired through field investigations and aircraft-borne instruments, Fischer and her colleagues are exploring how a trace gas called peroxyacetyl nitrate (commonly known as “PAN”) impacts the distribution of ozone in the troposphere.
In the lower atmosphere, PAN and ozone are two important pollutants, particularly in and around urban areas. Both are poisonous to plants in high concentrations, and both can affect human health. PAN is a strong eye and respiratory irritant, and ground-level ozone can cause a wide range of respiratory issues. As noted by the U.S. Environmental Protection Agency (EPA), “long-term exposure to [low-level] ozone is linked to aggravation of asthma, and is likely to be one of many causes of asthma development. Long-term exposures to higher concentrations of ozone may also be linked to permanent lung damage.”
Tropospheric ozone forms in a different way than stratospheric ozone. In the troposphere, nitrogen dioxide (NO2) rather than molecular oxygen (O2) is the primary source of the oxygen atoms required for ozone formation. Sunlight splits nitrogen dioxide (NO2) into nitric oxide (NO) and an oxygen atom (O), and this oxygen atom combines with molecular oxygen to form ozone (O + O2 = O3).